Adapting Bloodculture Systems to Monitor Antimicrobial Efficacy

Principle Investigator: Irene Zaghi, M.D.
Supervisors:
- Russell E. Lewis, Pharm.D., University of Padua
- Monica Cricca, M.D., Ph.D., University of Bologna
- Vittorio Sambri, M.D., Ph.D., Univeristy of Bologna
Antibiotic dosing is an uncertain science

Antibiotic dosing is an uncertain science
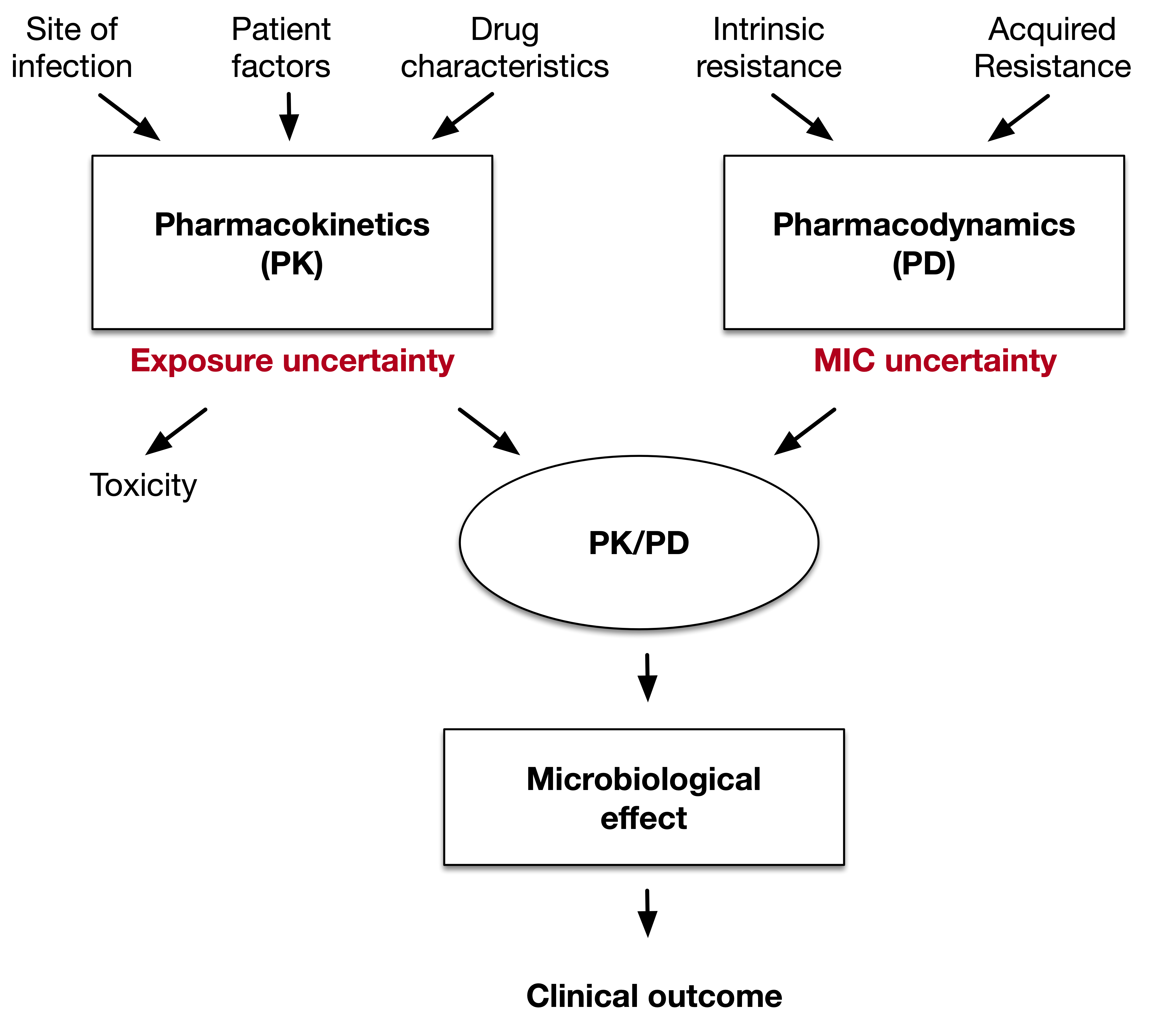
Antibiotic dosing is an uncertain science
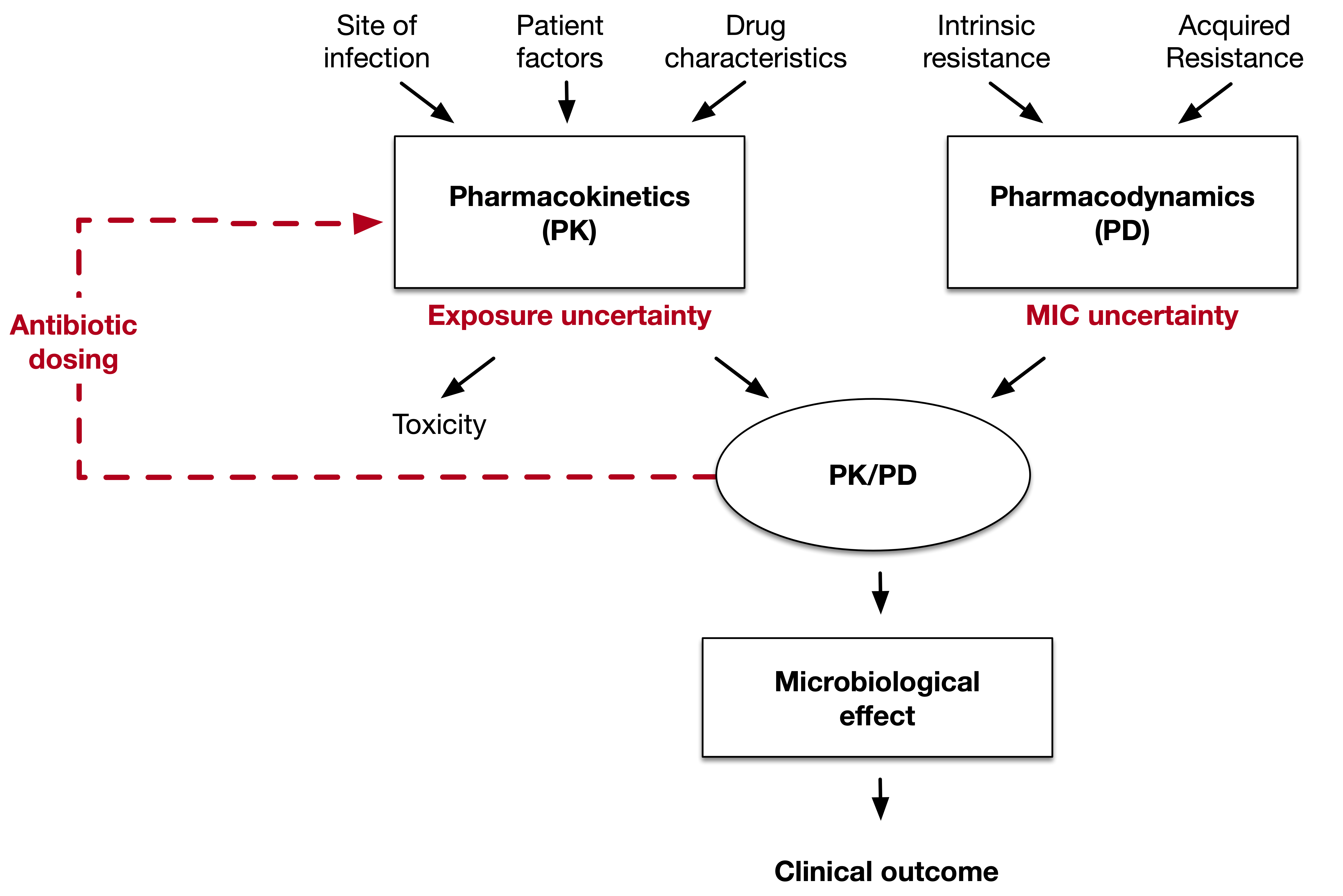
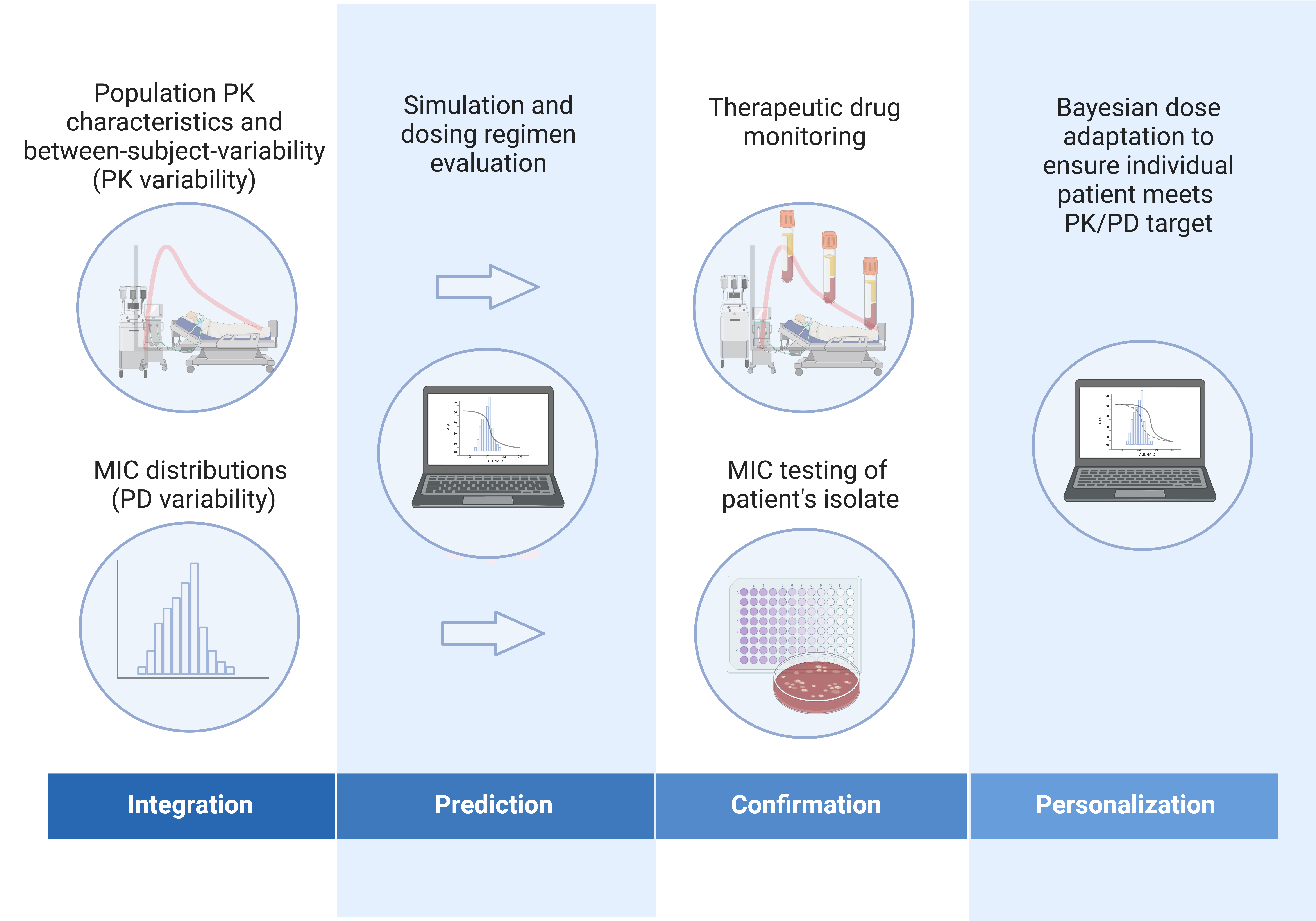
How can dosing uncertainty be managed?
Dosing uncertainty at the dawn of penicillin resistance

Dosing uncertainty at the dawn of penicillin resistance
“It was felt that there was a need of some definite basis for determining adequate therapy for each specific infectious organism involved…”
“Current methods in general use all depend on indirect comparisons (MICs, measurement of penicillin blood levels). In patients with more resistant organisms, it has not been demonstrated that this is the proper approach…”
“…It occurred to us that a more satisfactory method of outlining adequate therapy in resistant organisms might be based on the action of the patient’s own serum during penicillin administration.”
Serum bactericidal test (SBT)
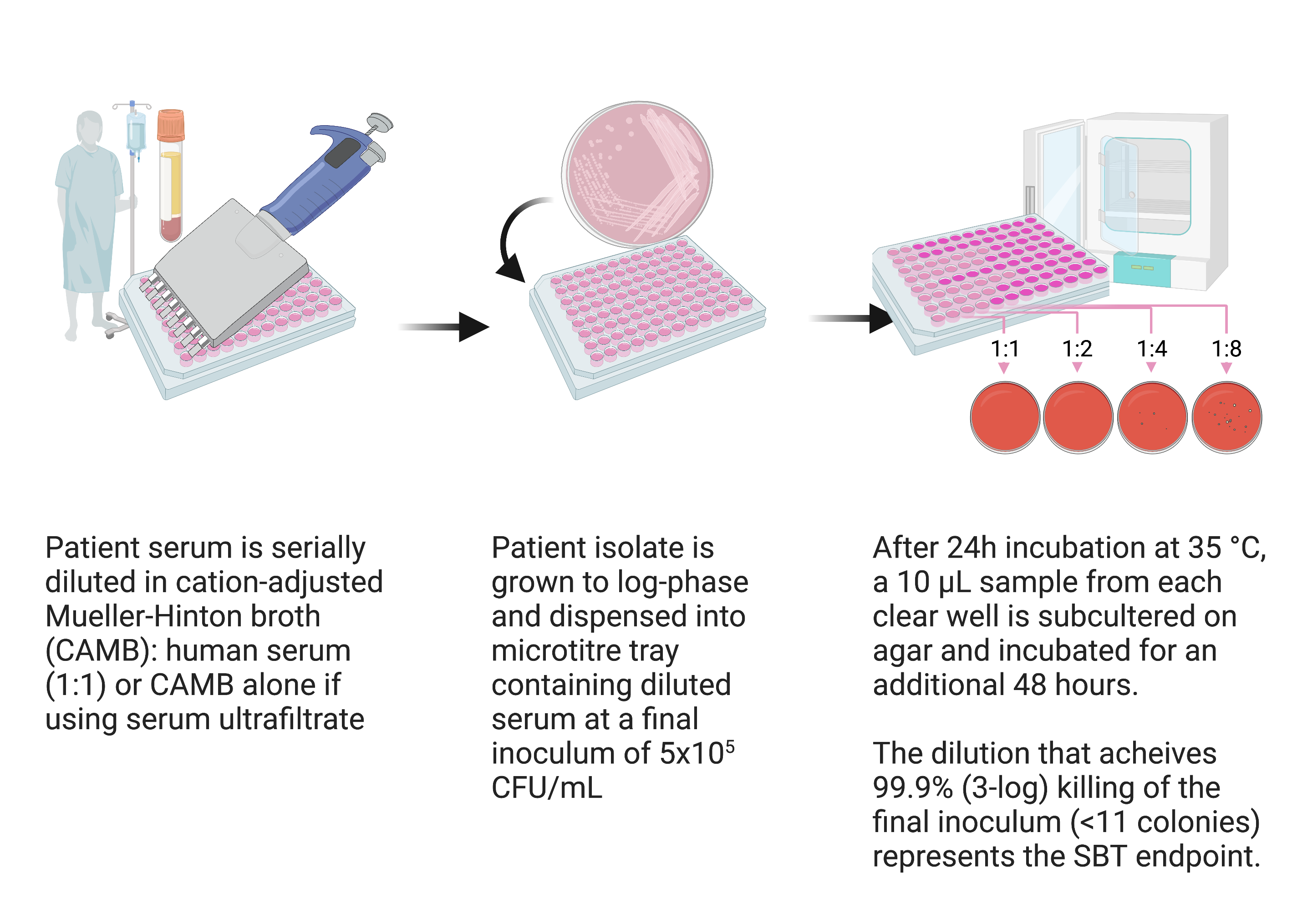
SBTs correlate with antibiotic PK/PD
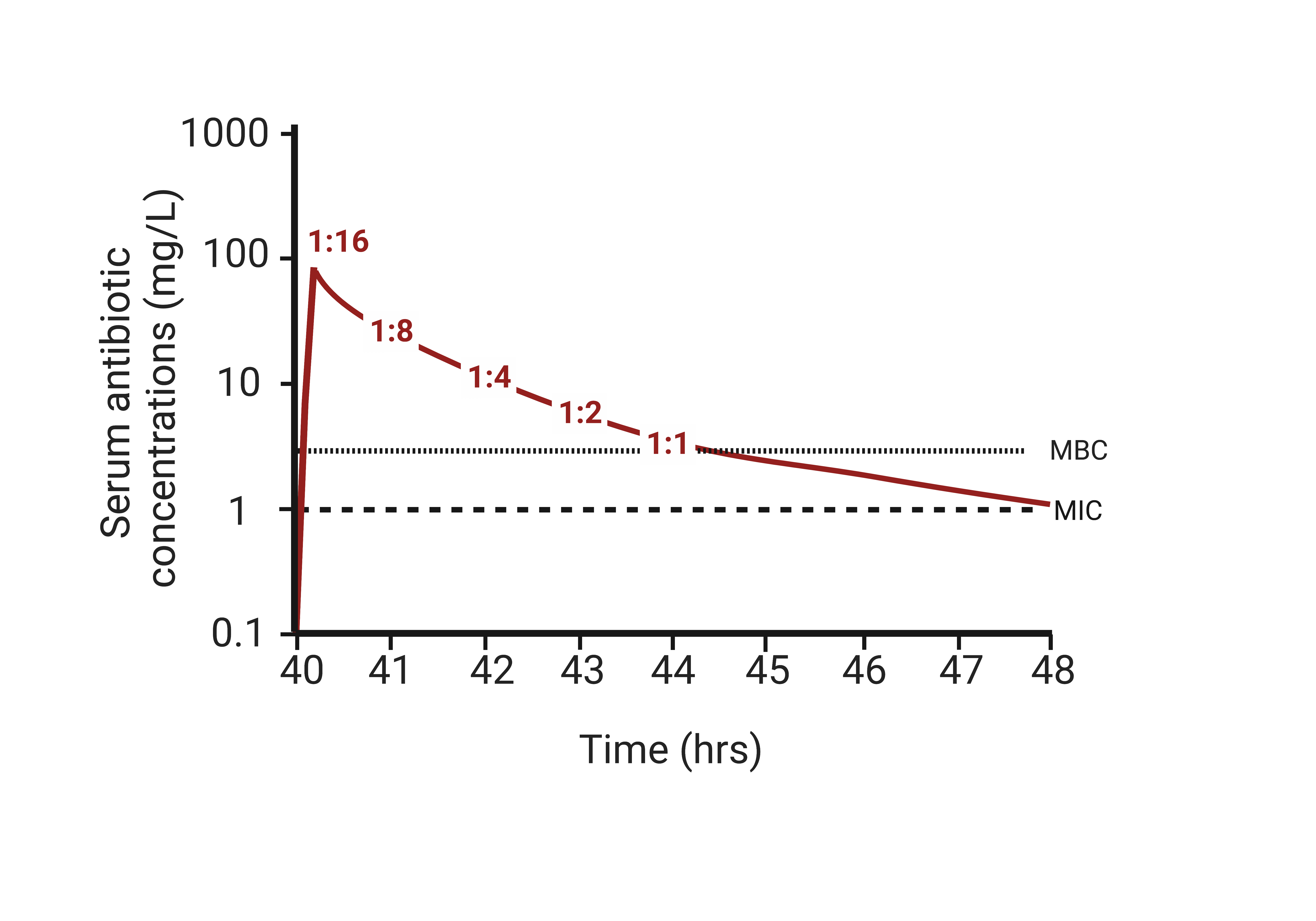
Potential clinical utility of SBTs
Individual patient-level diagnostic meta-analysis (1947-2020)
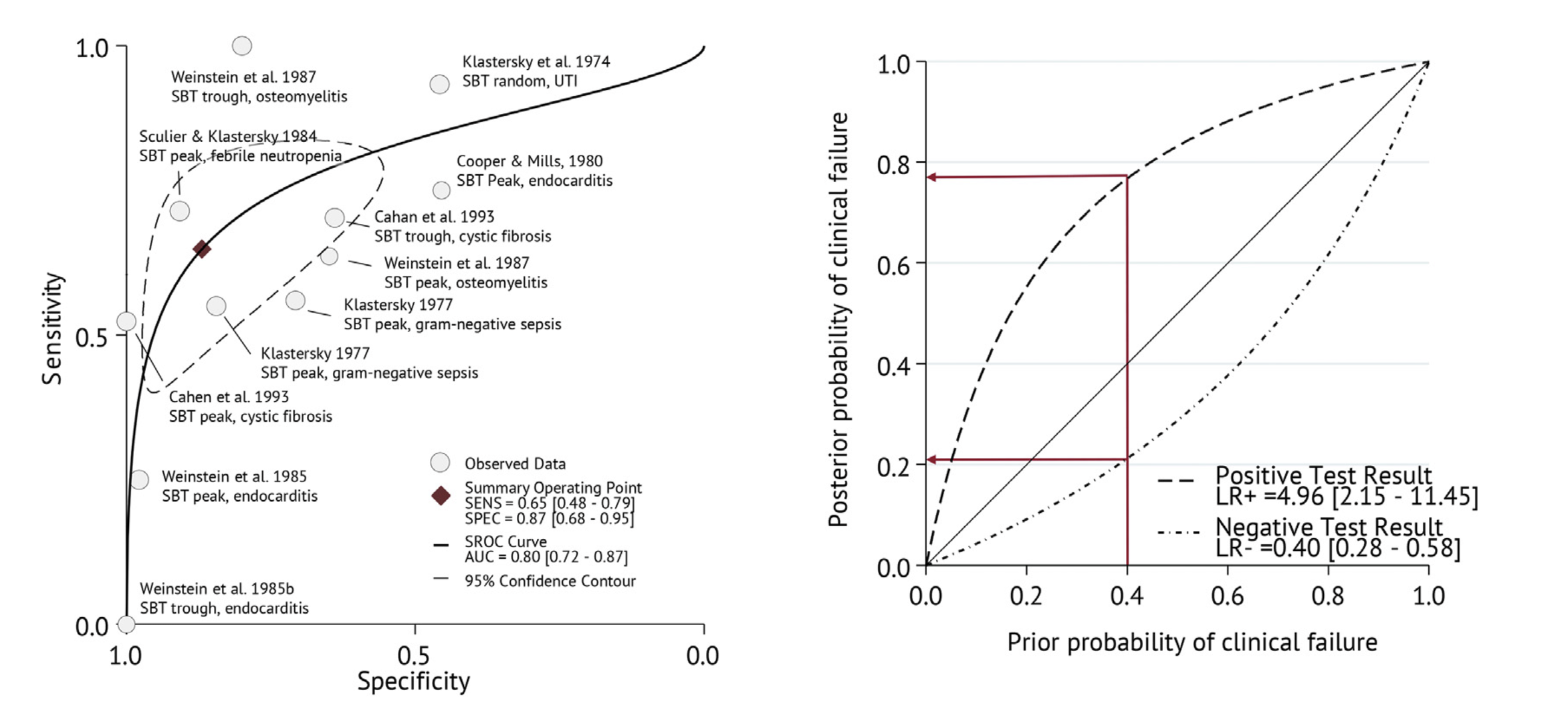
Prognostic performance similar or better than current susceptibility breakpoints
SBT advantages
- Only microbiological test that accounts for both antibiotic PK and PD
- Accounts for protein binding changes
- Can detect synergistic or antagonistic antibiotic interactions
Why were SBTs abandoned?
- Insufficient standardization despite published methods
- CLSI M21-A, last updated 1999
- Poor inter-laboratory reproducibility
- Labour-intensive to perform
- Lack of perceived need after introduction of reliably bactericidal antibiotics in 1990s
- 3rd generation cephalosporins, fluoroquinolones, carbapenems
- Delay in results reporting (48-72 hours with patients isolate)
“I read your SBT review and have a suggestion…”
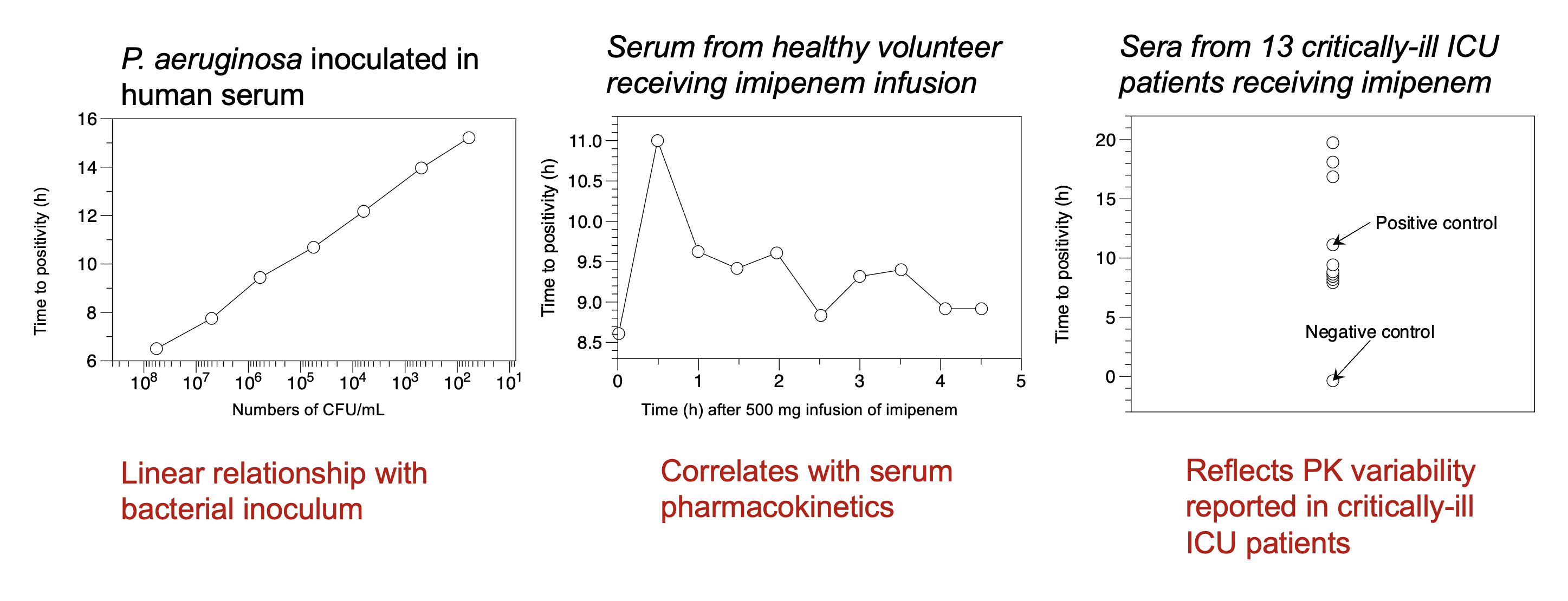
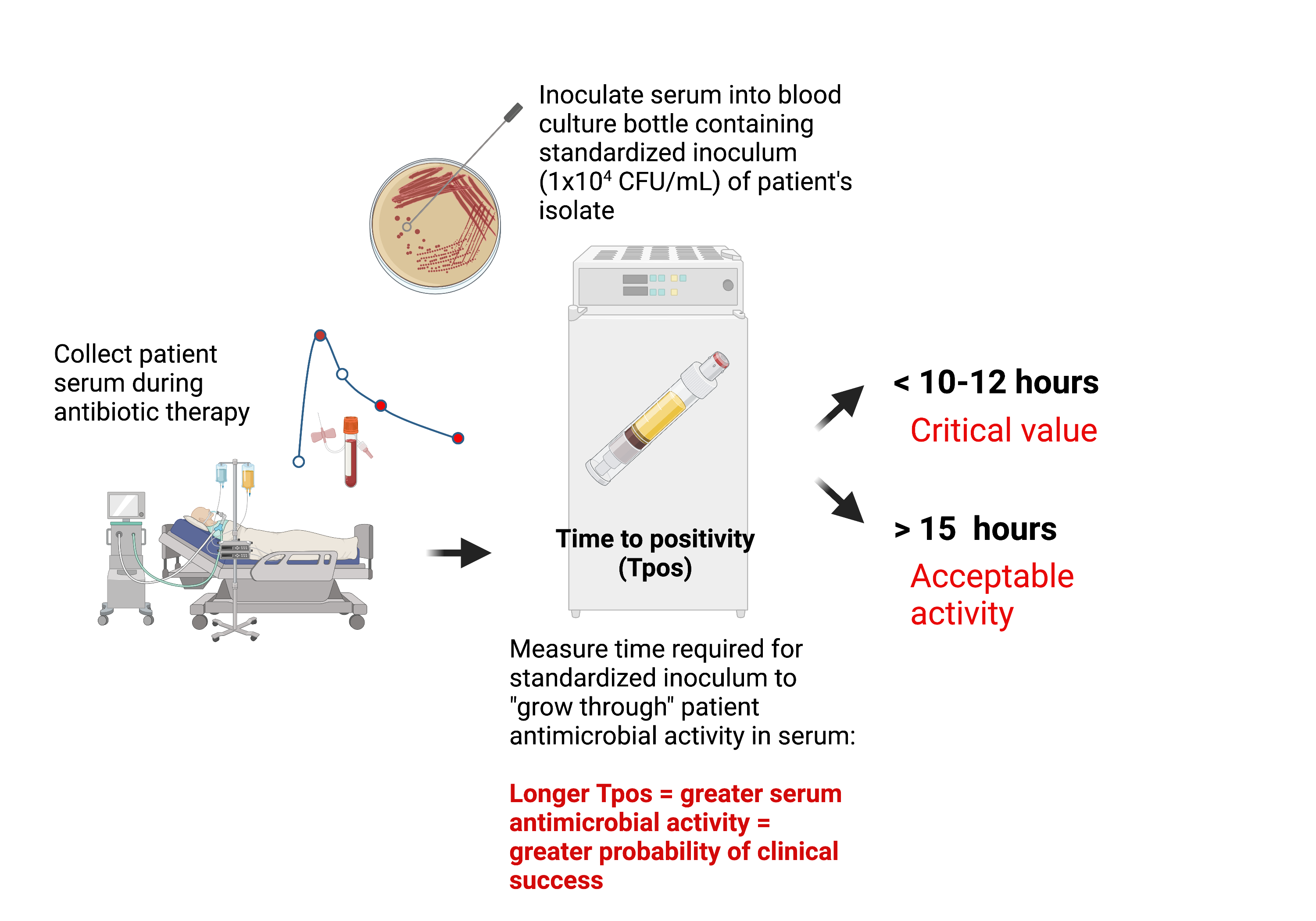
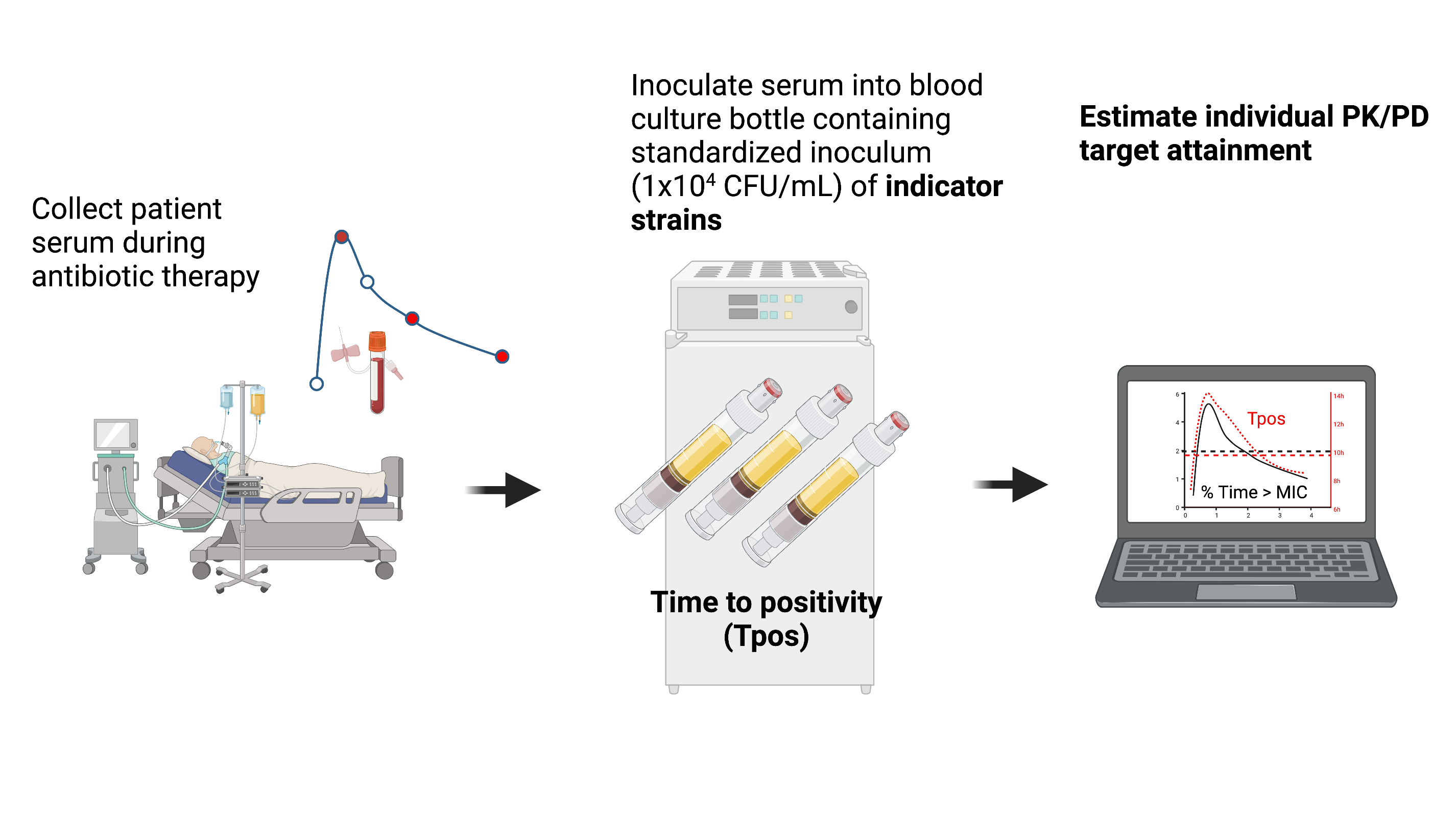
Use continuously-monitored bloodculture systems Time-to-positivity (Tpos) to measure bactericidal activity
Concept: The assay will measure time taken for a standardized bacterial inoculum to “grow through” the antimicrobial activity in the patient’s serum under defined conditions.

Blood culture Tpos measures bacterial inoculum and antimicrobial pharmacokinetics
Adapting Bloodculture Systems to Monitor Antimicrobial Efficacy

- Specific Aim# 1: Establish the quantitative relationship in vitro between Tpos and carbapenamase-producing Enterobacterales (CPE) and define antibiotic concentration-effect relationships
- Specific Aim#2: Pilot observational clinical study in 20 septic patients undergoing treatment for CPE to explore how early Tpos results with “indicator” isolates correlate with individual antibiotic PK/PD targets
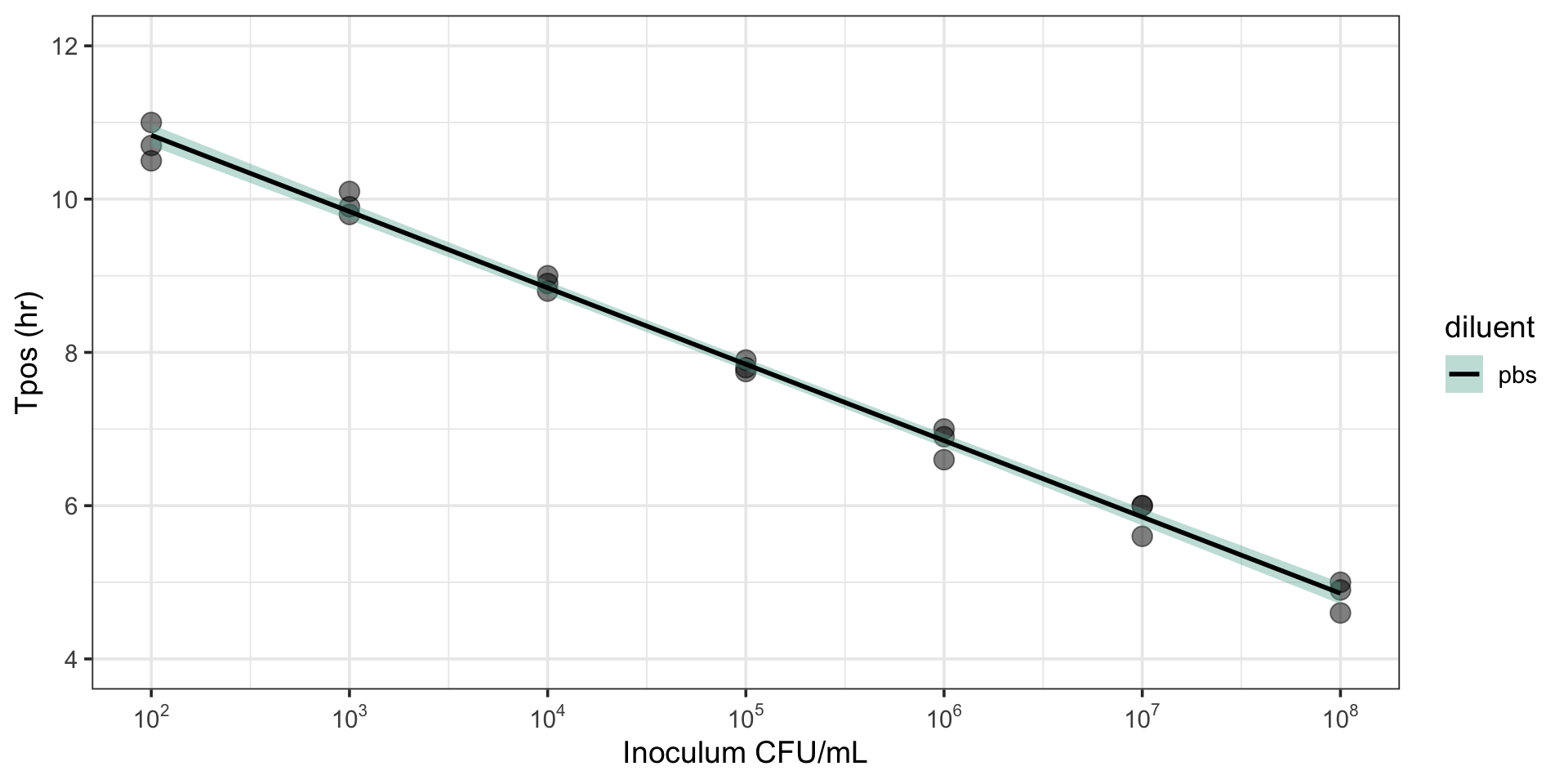
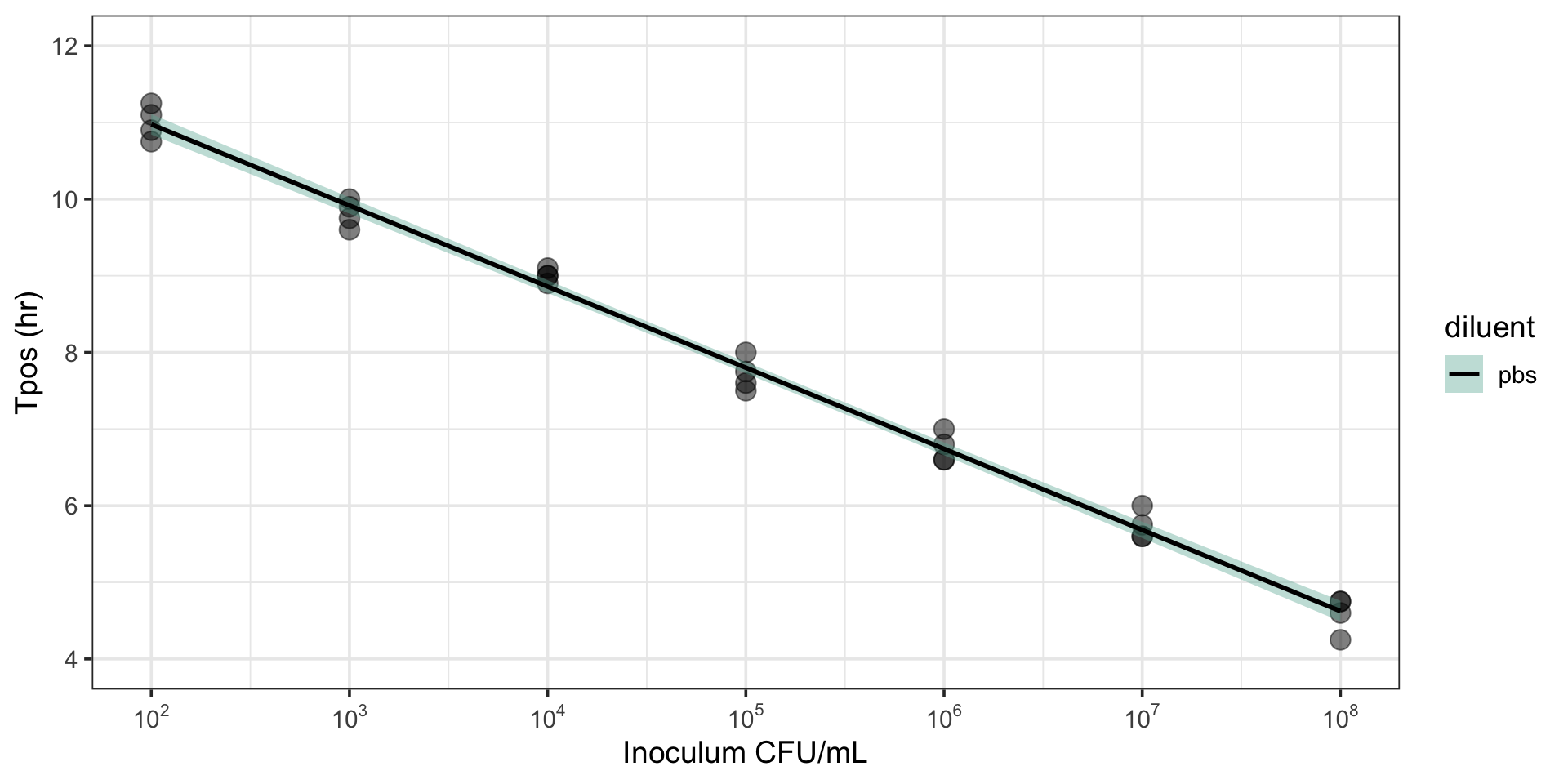
Tpos correlates with inoculum
R2 = 0.96-0.99
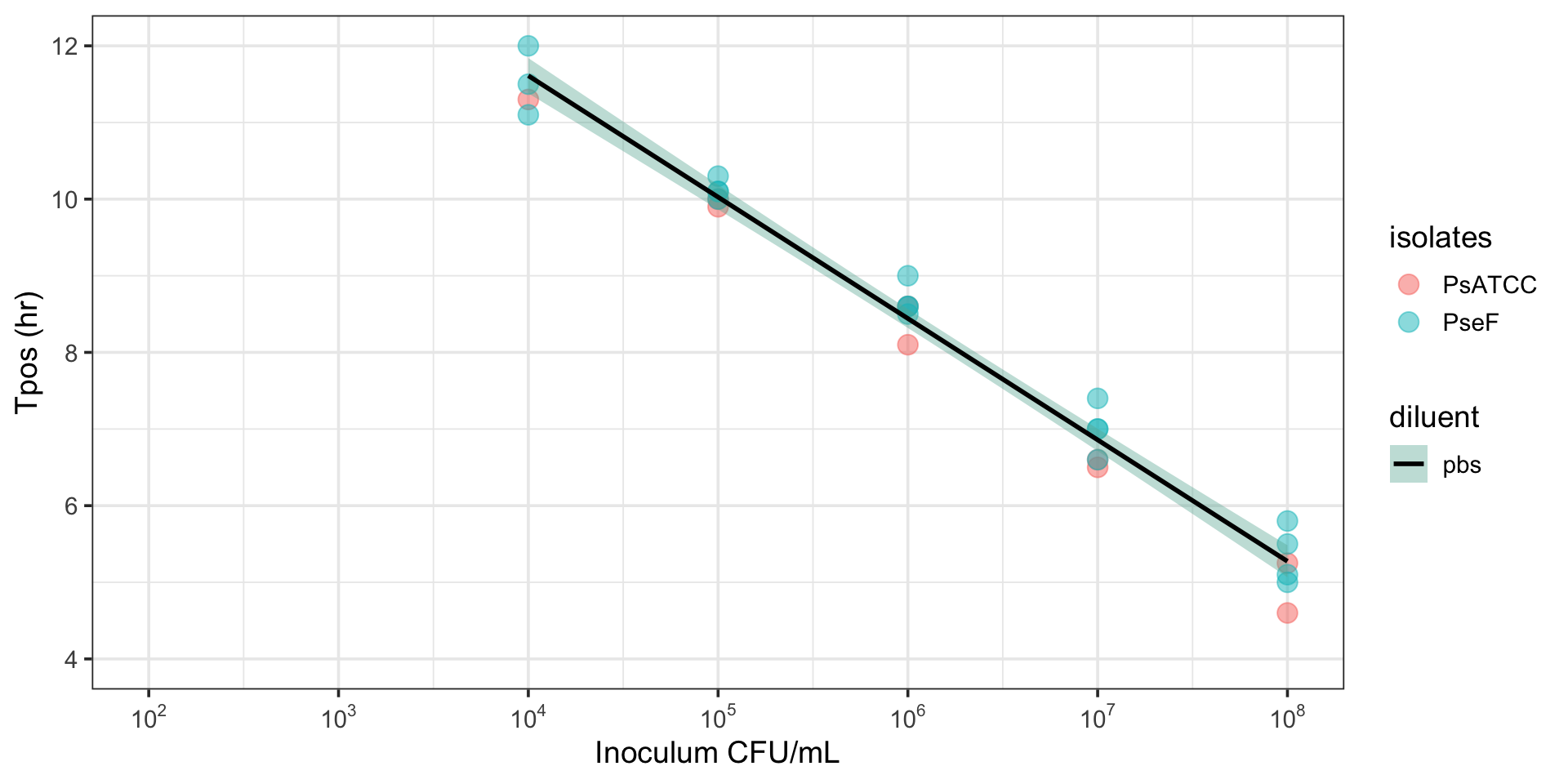
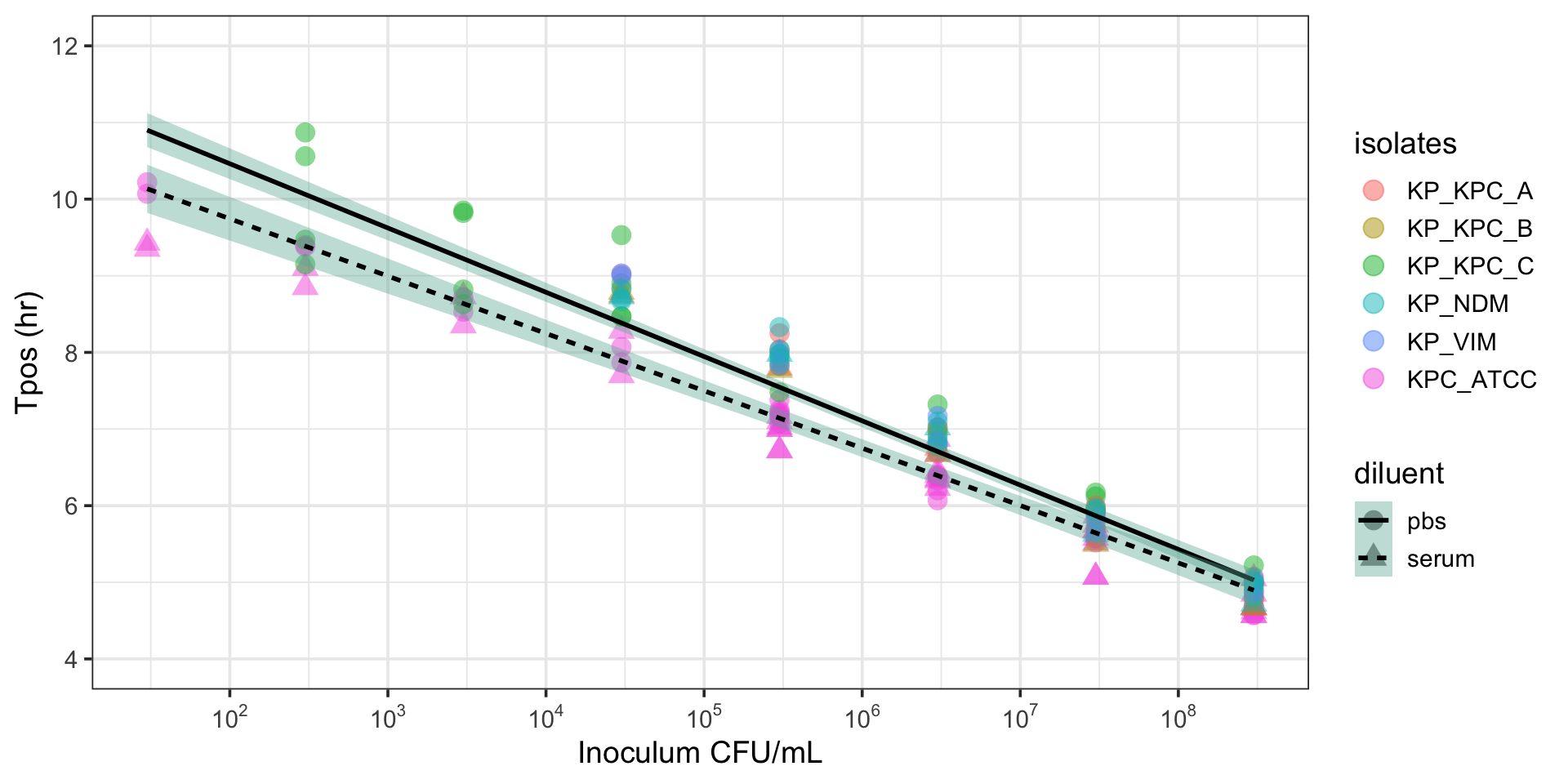
Tpos can discriminate between susceptible and resistant pathogens
Pharmacodynamic relationship of Tpos against
K. pneumoniae ATCC 700603 (0.75 mg/L)
| Estimate | Lower .95 | Upper .95 | |
|---|---|---|---|
| 0.1 | 23.87874 | 19.31716 | 28.44033 |
| 0.25 | 26.93812 | 24.06902 | 29.80723 |
| 0.5 | 30.38948 | 29.11007 | 31.66888 |
| 0.75 | 34.28302 | 31.38838 | 37.17767 |
| 0.9 | 38.67541 | 33.84814 | 43.50268 |
| 0.95 | 41.98020 | 33.98412 | 49.97627 |
| Estimate | Lower .95 | Upper .95 | |
|---|---|---|---|
| 0.1 | 8.099087 | 7.038485 | 9.159689 |
| 0.25 | 9.235833 | 8.728989 | 9.742676 |
| 0.5 | 10.532127 | 10.149119 | 10.915134 |
| 0.75 | 12.010361 | 10.856802 | 13.163921 |
| 0.9 | 13.696074 | 11.685276 | 15.706872 |
| 0.95 | 14.975851 | 12.415855 | 17.535846 |
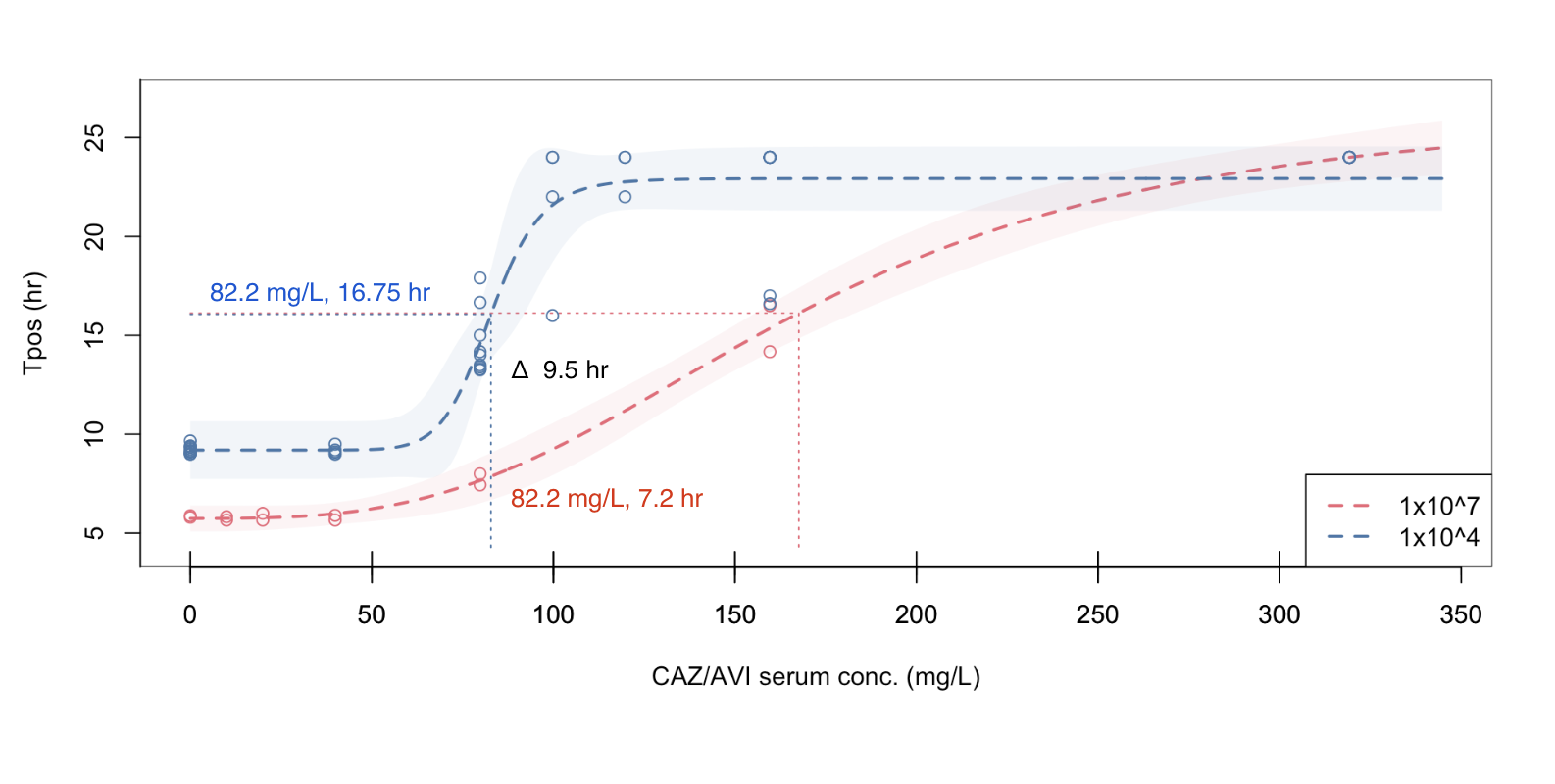
Inoculum effects observed with PD relationship measured by Tpos
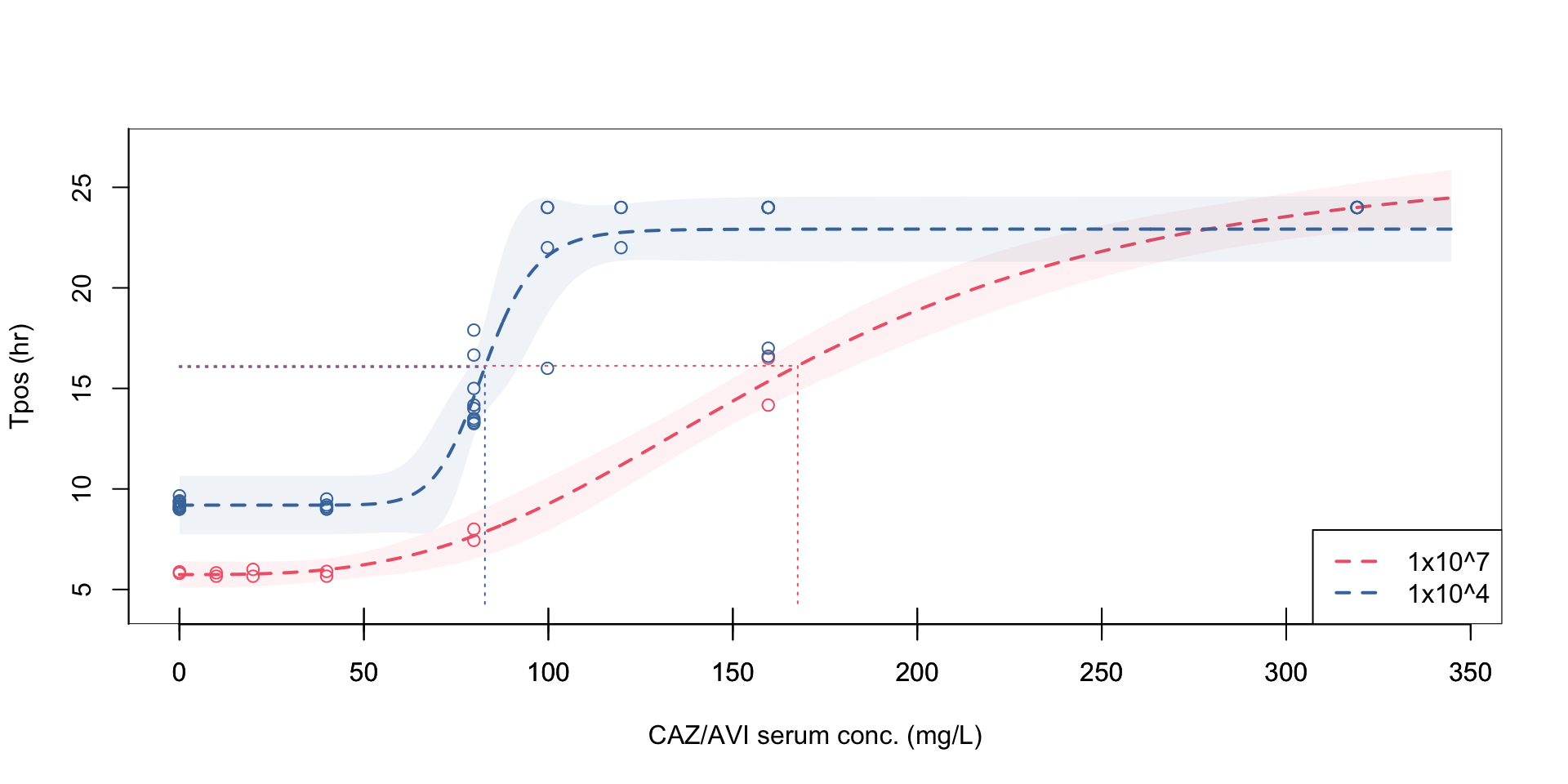
At a standardized inoculum, Tpos demonstrates a consistent relationship across different antibiotics and isolates with different MICs
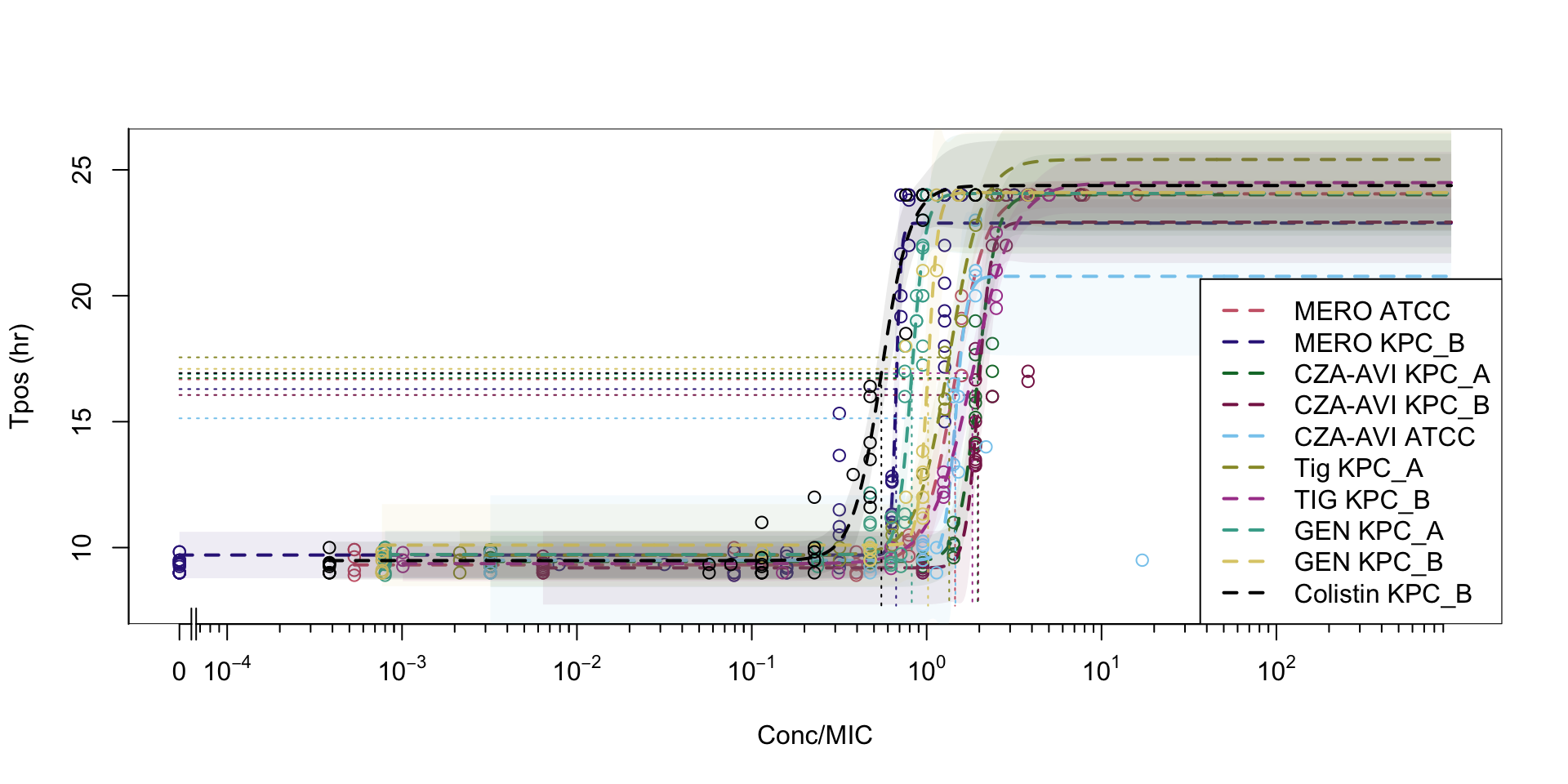
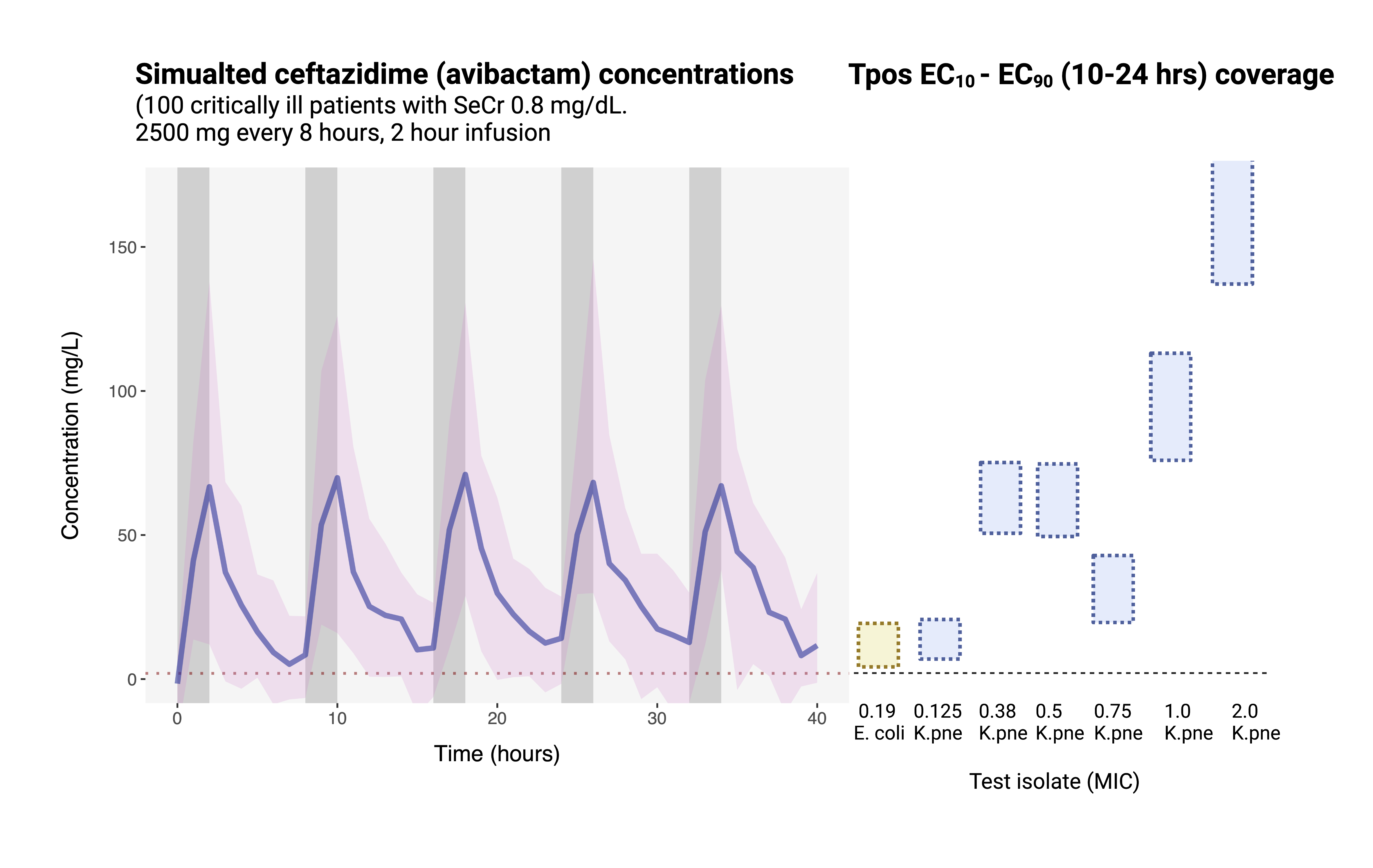
Tpos for directed antimicrobial therapy “in vivo MIC”
Tpos performed using indicator isolates to screen for suboptimal PK/PD
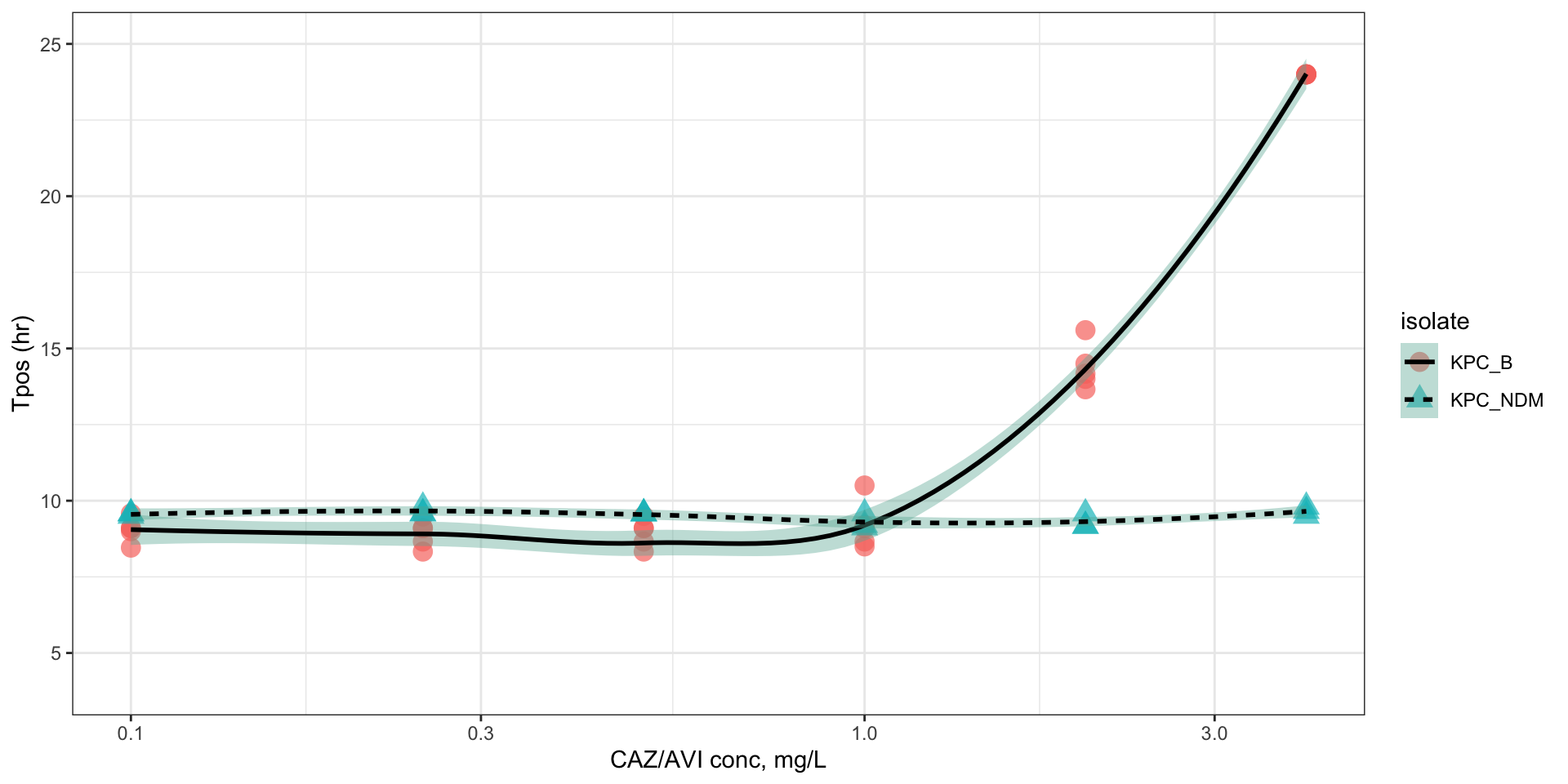
Comparison of K. pneumoniae indicator isolates
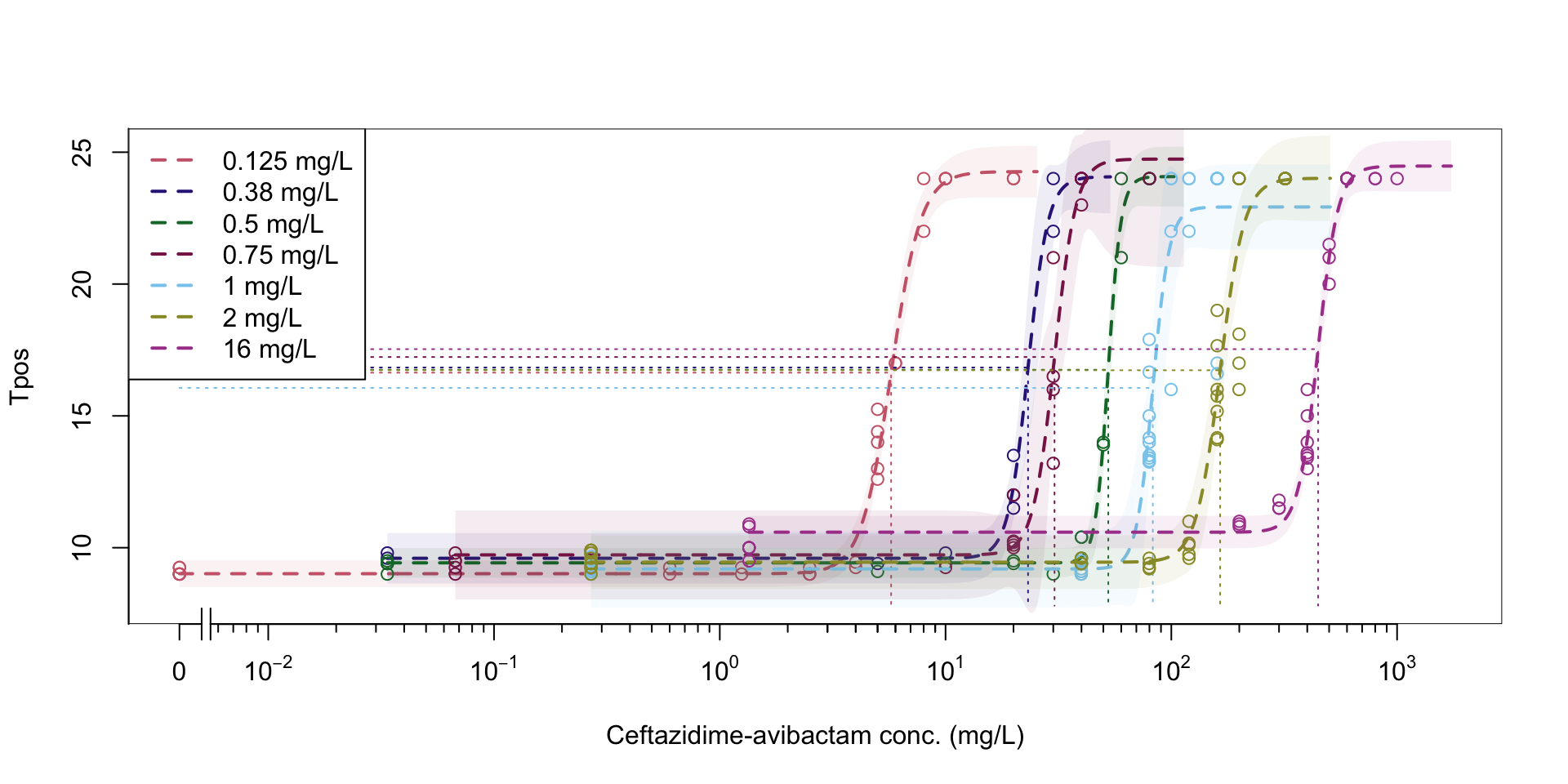
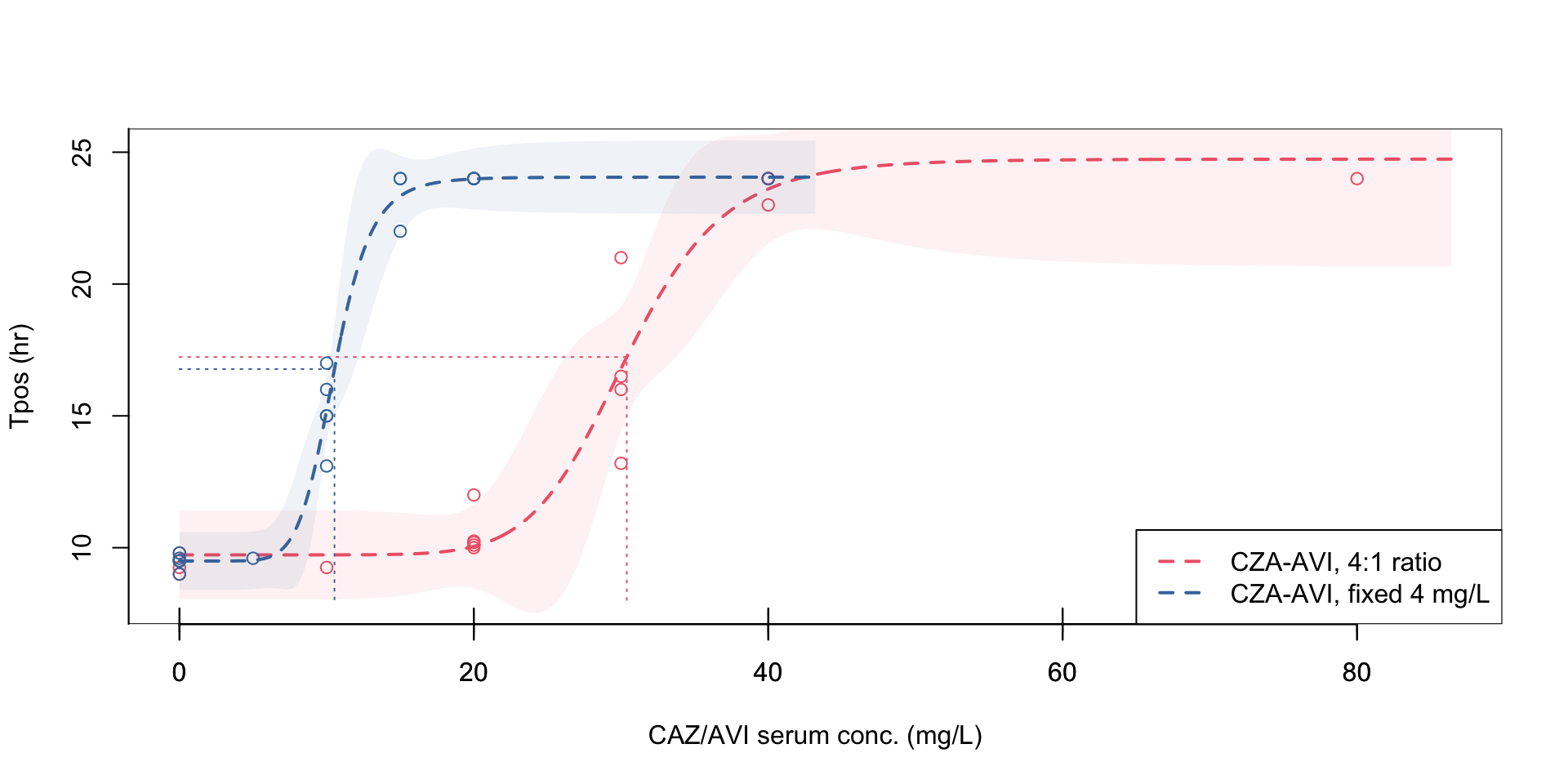
Figure 1: Pharmacodynamic relationship of Tpos to ceftazidime/avibactam concentrations
Isolate PD coverage of ceftazidime-avibactam
Combination therapy
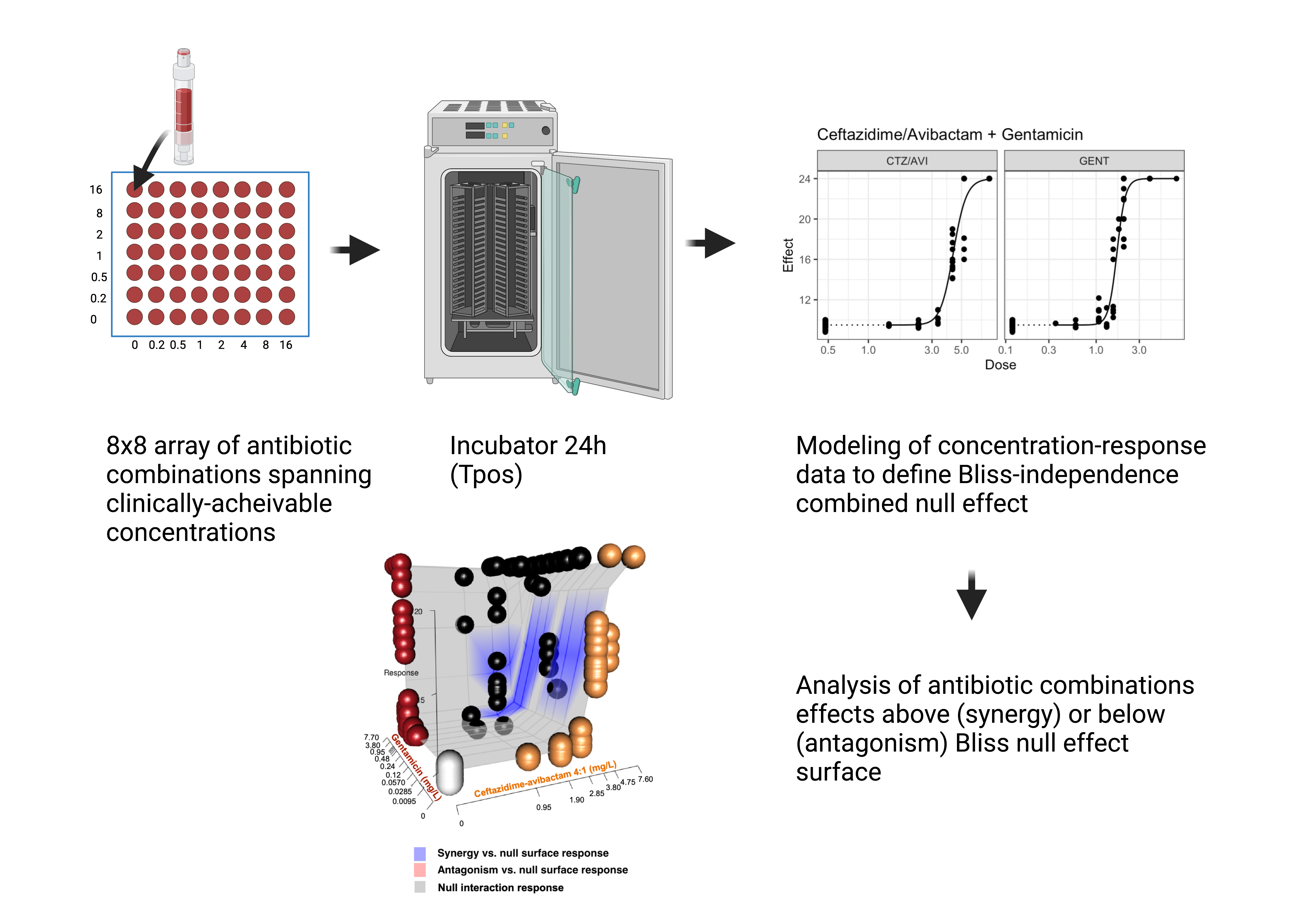
Synergy of ceftazidime-avibactam plus gentamicin versus KPC-producing K. pneumoniae
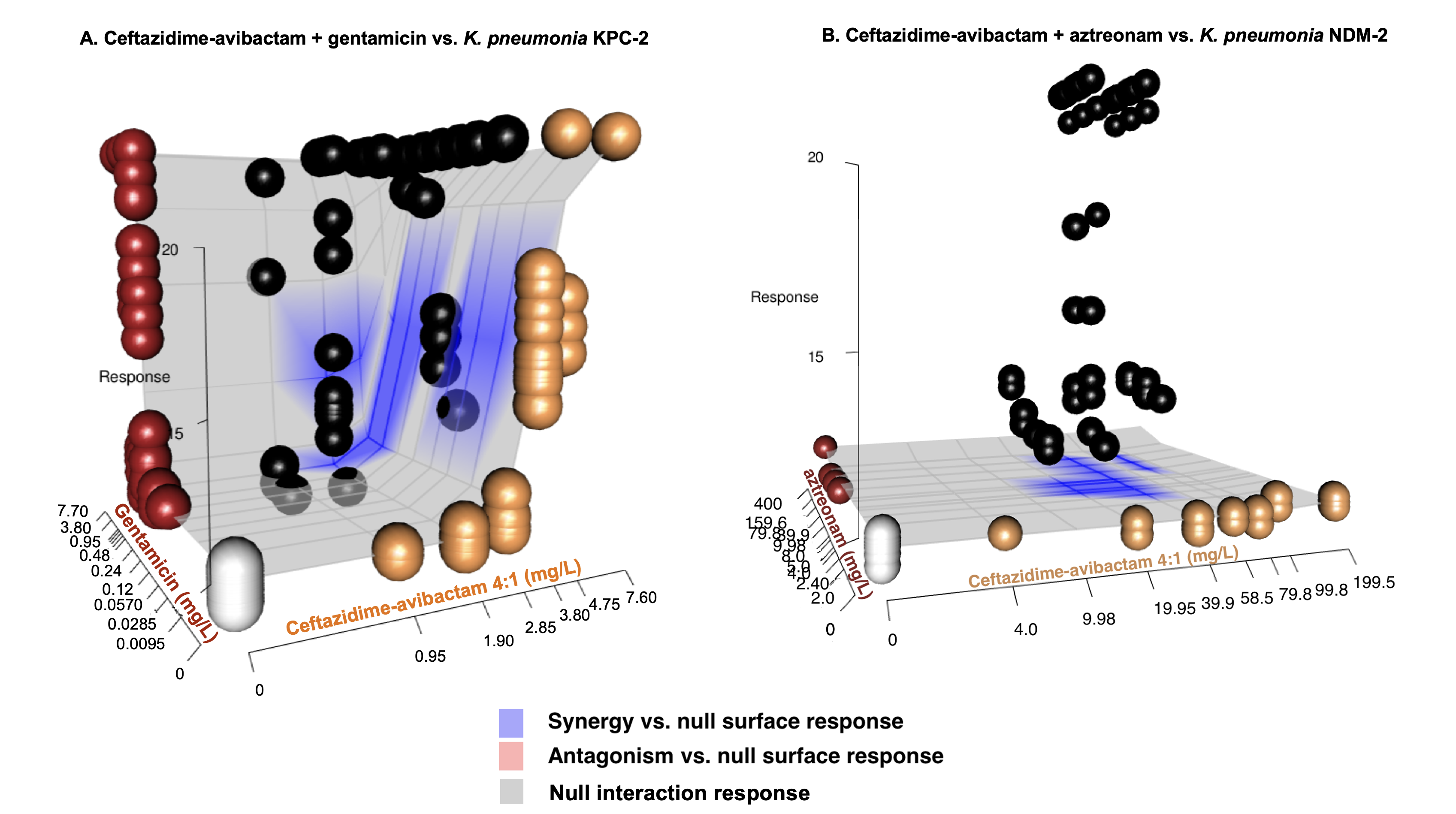
Combination therapy versus Klebsiella pneumoniaeo
| Antibiotic combination | Strain (Ceftazidime-avibactam MIC mg/L) |
Mean Tpos change (hr) from predicted null response surface (95% CI) |
|---|---|---|
| CZA + GENT | KP_A; KPC (2) | +5.68 (5.09-6.53) |
| CZA + GENT | KP_B; KPC (1) | +3.05 (2.16-4.03) |
| CZA + GENT | KP_Catania; KPC (16) | +3.23 (2.10-4.14) |
| CZA + COL | KP_B; KPC (1) | +2.31 (1.40-3.20) |
| CZA + TGC | KP_B ;KPC (1) | +1.66 (0.72-2.73) |
| CZA + ATM | KP_NDM;NDM-2 (> 64) | +10.33 (10.32-10.34) |
| CZA + ATM | KP_Catania; KPC (16) | +10.36 (10.31-10.35) |
| CZA + MER | KP_B; KPC (1) | +11.61 (11.05-11.95) |
| CZA + MER | KP_Catania; KPC (16) | +3.04 (2.15-4.03) |
Impact of combination therapy on high-inoculum infections
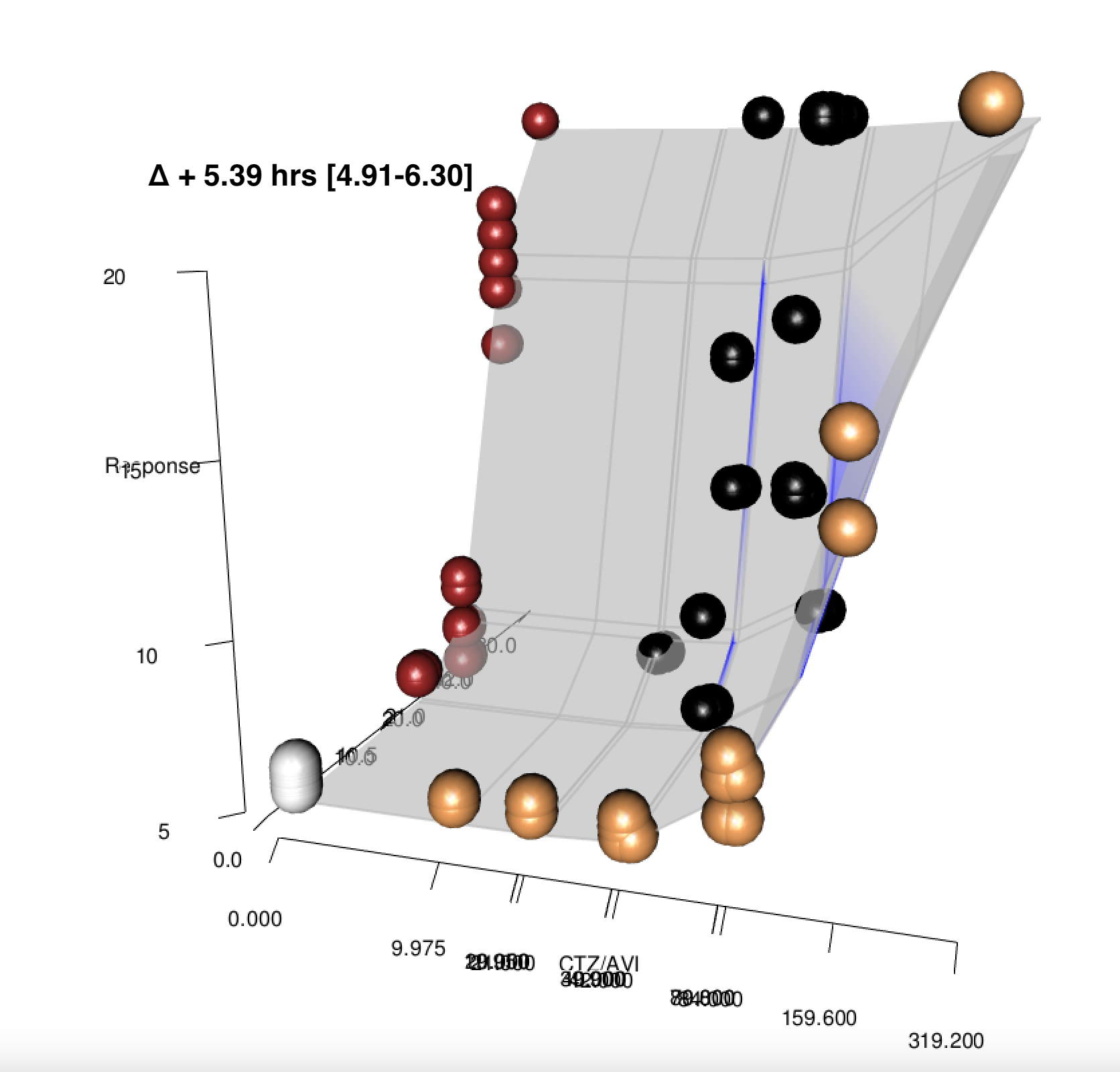
synergistic activity improved but did not overcome effects of high-inoculum infection
Adapting Bloodculture Systems to Monitor Antimicrobial Efficacy

Specific Aim# 1: Establish the quantitative relationship in vitro between Tpos and carbapenamase-producing Enterobacterales (CPE) and define antibiotic concentration-effect relationships
Specific Aim#2: Pilot observational clinical study in 20 septic patients undergoing treatment for CPE to explore how early Tpos results with “indicator” isolates correlate with individual antibiotic PK/PD targets
Longer range goals
- Develop population PK/PD tools for interpreting and predicting Tpos with ceftazidime-avibactam and other core antibiotics
- Expand Tpos analysis for other difficult to treat Gram-negative pathogens (e.g., Acinetobacter baumanii, Pseudomonas aeruginosa) and Candida spp.
- Future multicentre collaborative trials (validation, testing)
- Pilot projects to develop Tpos as dosing tools in LMICs?
Acknowledgements
Supervisors:
- Prof. Russell Lewis, Department of Molecular Medicine, University Of Padua
- Prof. Monica Cricca, Department of Diagnostic and Experimental Medicine, University of Bologna
- Prof. Vittorio Sambri, Department of Diagnostic and Experimental Medicine, University of Bologna
- Faculty and staff at the Microbiology Unit, Greater Romagna Area Hub, Pievesistina, Cesena, Italy
Funding:
Direzione Generale Della Ricerca Dell’ Innovazione in Sanità 2021-Bando Dell Ricerca

European Society of Clinical Microbiology and Infectious Diseases (ESCMID) 2022 Research Grants Program

Antibiotic powder: